It is all about expectations

Today early drop-out still is a major challenge we must deal with when running clinical trials. The past few years patient centricity and patient engagement initiatives have generated major improvements. This is the first in a series of articles about a different perspective on the subject and a new and innovative approach to improve early drop-out rates.
Early Drop-out; still a major challenge
In 2018 global spending on drug development is expected to reach $172 billion[1] and that number will hit $204 billion by 2024. Clinical studies account for 45-75% of these costs[2]. In 2012 the life sciences industry was already spending $1.2 billion[3] on patient recruitment alone! Of these studies 85% fail to retain enough patients[4] and 95% fail to meet the original timeline and budget[5].
Early drop-out is a major challenge when running clinical trials. Early drop-out rates are 30%[6] on average and they have a very negative impact on site level time lines. At these rates drop-outs can and will disrupt the overall planning and logistics of the trial, resulting in unacceptable costs of health care.
Over the past few years the health care industry has made considerable steps forward to better align clinical trials to the needs and wants of patients. True patient engagement by involving patients from the earliest start of the clinical trial has led to more effective and feasible clinical trial protocols. The Informed Consent process is focused on making trial information much more accessible and understandable to the general public.
Early drop-out rates are improving, but is there a way to do even better?
It is all about expectations
A patient can have many reasons to drop-out, but previous studies have shown that these are the most common reasons.
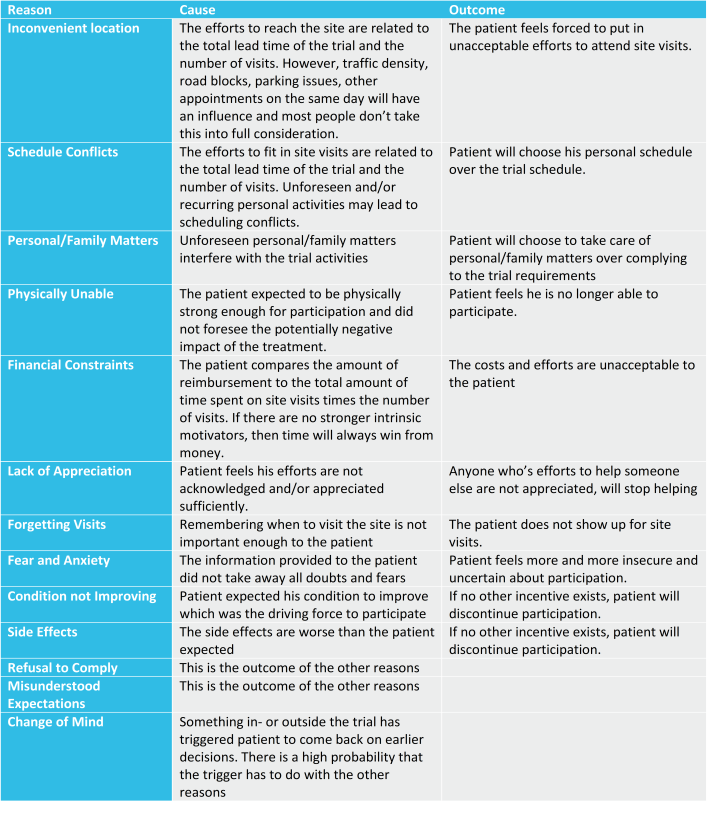
All information that should prevent the patient from dropping out early because of these reasons, is shared during the Informed Consent process. The language used is aligned to the patients’ capabilities. And finally, the patient has acknowledged that he understands all information conveyed.
However, when we take a closer look at these reasons for early drop-out than we can only conclude that misconceptions and wrong expectations are the main reasons why patients drop out during study conduct. This is very surprising considering the amount of effort put into making trial information as clear and understandable as possible to the patient. The early drop-out rates prove that despite all efforts communication is not as effective as it should be.
Could it be that when communicating with patients, we are missing out on something essential? Is there a better, more effective way to communicate and manage patient expectations?
Read our next article on the subject and please do not hesitate to provide your take and input in the comments below!
[1] EvaluatePharma, World Preview 2018, Outlook to 2024, June 6, 2018
[2] http://www.pfizer.com/research/clinical_trials/phases_of_development
[3] Tufts CSDD, 2010 Survey of 3,516 Global Sites and 2012 survey of 48 sponsors and CROs
[4] Aidan Nuttall 2012-03, Considerations For Improving Patient Recruitment Into Clinical Trials 2012-03
[5] Tufts Center for the Study of Drug Development Impact Report 15/1“New Research from Tufts Characterizes Effectiveness and Variability of Patient Recruitment and Retention Practices”, January/February 2013
[6] Applied Clinical Trials, RbM Guidance Document: Ten Burning Questions about Risk-Based Study Management, January 13, 2015
Like or Share
Choose category
Latest posts
- 04 November, 2025
- Link2Trials Q&A_30
- 15 August, 2025
- Link2Trials Q&A_29
- 18 July, 2025
- Link2Trials Q&A_28
- 04 July, 2025
- Link2Trials Q&A_27
- 03 June, 2025
- Link2Trials Q&A_26

 Argentina
Argentina Australia
Australia Balgarija
Balgarija België
België Canada
Canada Česko
Česko Chile
Chile China (中国)
China (中国) Colombia
Colombia Danmark
Danmark Deutschland
Deutschland England
England España
España France
France Ireland
Ireland Italiana
Italiana Lietuva
Lietuva Magyarország
Magyarország Nederland
Nederland New Zealand
New Zealand Österreich
Österreich Polska
Polska Schweiz
Schweiz Singapore
Singapore Slovenija
Slovenija Slovensko
Slovensko Suomi
Suomi Sverige
Sverige United States
United States Israel
Israel


