Why you should focus on retention

Growing costs of drug development
Over 95% of the clinical studies fail to meet their original time lines and budgets.[1] Between 2006 and 2016 the global spending on drug development grew from $108 billion to over $148 billion.[2] Clinical trials account for 45% to 75% of this cost[3] and in 2012 the life sciences industry was already spending $1.2 billion[4] on patient recruitment alone!
It is a well-known fact that delays and difficulties with patient recruitment have a tremendous negative effect on the costs and time lines of clinical studies. But even when we have overcome these challenges; we still need to deal with the early drop-out rates that can be quite high depending on the type of study.
Minimizing early drop-out rates is key
Misconceptions, wrong expectations and excessive burden are the main reasons why patients drop out during study conduct. Early drop-out rates are 30% on average[5] and they have a very negative impact on site level time lines. At these rates drop-outs can also disrupt the overall planning and logistics of the trial.
The financial consequences of all of this are tremendous. The cost per lost patient can run up to $6.322 but the indirect effects are much costlier than this. Each day a trial is delayed can cost $1.6 million lost revenue[5].
However, the biggest risk lies in the missing data[6] linked to patients who discontinue the assigned treatment because of adverse events, lack of tolerability, lack of efficacy, or simple inconvenience. Valuable information is lost if this data cannot be retrieved.
Patient centric retention services through SEHM
At Link2Trails we believe that lower drop-out rates can be achieved by a more patient centric approach.
Early 2016 we have taken the first steps to incorporate the Subjective Experienced Health Methodology (SEHM) in our retention services. SEHM makes it easy for your clinical staff to focus on patients who are in need of a truly patient centric way of communication and interaction.
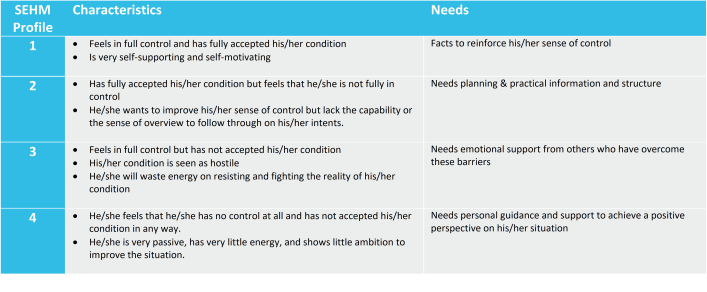
SEHM is initially developed at the Nyenrode Business University, The Netherlands by Prof Sjaak Bloem[7]. The purpose of SEHM is to better align the type of medical products and services offerings to the actual needs and wants of a patient at a specific point in time. It already has been successfully put into practice and fine-tuned for oncology, hematology, infectious diseases, diabetes and pulmonary diseases.
SEHM is based on three building blocks;
- a set of personality/mood types (SEHM profiles),
- a simple questionnaire to quickly and regularly determine each patient’s present SEHM profile,
- a set of communication and interaction guidelines per SEHM profile.
Everyone has experienced that your mood can swing from confident to insecure depending on the situation you are in. And we all know that if you feel insecure you are reluctant to stay in a risk full environment. The only way you can be persuaded to stay is when someone or something restores your confidence. SEHM will help you to focus on these individuals in your study population who are prone to drop-out soon.
SEHM is the most effective when it is integrated in the study from the very first beginning and all patients can be contacted directly by Link2Trials. Through a simple questionnaire we will determine each recruited patient’s baseline SEHM profile, and during study execution we will check regularly if this has shifted into another profile.
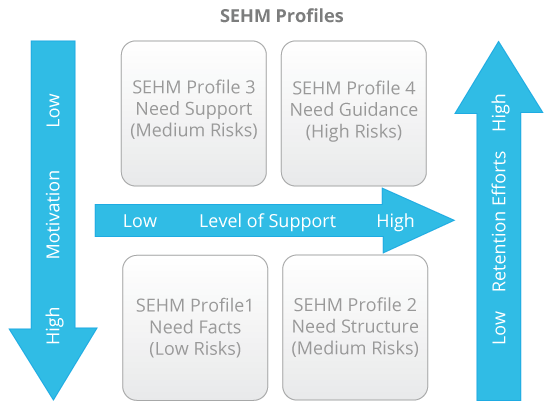
Based on the SEHM profile of a patient we will align our retention efforts and interactions. This could be pushing factual information about the study to a SEHM Profile 1, sending out visit schedules and reminders to a SEHM Profile 2, publishing testimonials from patients who have come to peace with the reality of their condition for a SEHM Profile 3, or providing hand-holding and personal coaching to a SEHM Profile 4.
Benefits of SEHM
SEHM will help you to quickly and easily implement patient centricity in your study.
The main benefits are:
- No training needed
- Easy to incorporate in your study
- Cost effective because of lower early drop-out rates
- Helps to focus on patients that are on the verge of dropping out
References
[1] Tufts Center for the Study of Drug Development Impact Report 15/1“New Research from Tufts Characterizes Effectiveness and Variability of Patient Recruitment and Retention Practices”, January/February 2013.
[2] EvaluatePharma, World Preview 2014, Outlook to 2020, June 23, 2014
[3]http://www.pfizer.com/research/clinical_trials/phases_of_development
[4] Tufts CSDD, 2010 Survey of 3,516 Global Sites and 2012 survey of 48 sponsors and CROs
[5] Applied Clinical Trials, RbM Guidance Document: Ten Burning Questions about Risk-Based Study Management, January 13, 2015
[6] The Prevention and Treatment of Missing Data in Clinical Trials, The New England Journal of Medicine, October 4, 2012
[7] Bloem, J.G. & Stalpers, J.F.G. (2012). Subjective experienced health as a driver of health care behaviour. Nyenrode research papers series no 12-01 (9 July). Nyenrode Business University, The Netherlands. p. 22
Like or Share
Choose category
Latest posts
- 04 November, 2025
- Link2Trials Q&A_30
- 15 August, 2025
- Link2Trials Q&A_29
- 18 July, 2025
- Link2Trials Q&A_28
- 04 July, 2025
- Link2Trials Q&A_27
- 03 June, 2025
- Link2Trials Q&A_26

 Argentina
Argentina Australia
Australia Balgarija
Balgarija België
België Canada
Canada Česko
Česko Chile
Chile China (中国)
China (中国) Colombia
Colombia Danmark
Danmark Deutschland
Deutschland England
England España
España France
France Ireland
Ireland Italiana
Italiana Lietuva
Lietuva Magyarország
Magyarország Nederland
Nederland New Zealand
New Zealand Österreich
Österreich Polska
Polska Schweiz
Schweiz Singapore
Singapore Slovenija
Slovenija Slovensko
Slovensko Suomi
Suomi Sverige
Sverige United States
United States Israel
Israel


