Recruitment
Blog » recruitment
How to Recruit Patients for Clinical Trials: A Comprehensive Guide
Simon Klaasen
5359

05-02-2025 15:49
How to Recruit Patients for Clinical Trials: A Comprehensive Guide
Recruiting patients for clinical trials is a crucial aspect of clinical research, yet it remains one of the most significant challenges in the industry. A well-executed recruitment strategy ensures that studies are completed on time, improves data reliability, and ultimately accelerates the development of new treatments....Read more
PSL (Patient Site Liaison) journey at Link2Trials
Stephanie Gontijo
2860
07-09-2022 12:57
My name is Stephanie Maria Gontijo dos Santos Lima and I want to share a bit about my journey in the last 4 years with Link2Trials.During the last years of my medical studies, I worked as a PSL for different clinical trials in different countries: from Spain, UK to USA and for studies recruiting people with conditions such as back pain, to diabetes or cancer. Working with recruiting...Read more
How to Improve Medical Device Clinical Trials With The Right Partner Ecosystem
Simon Klaasen
2869
01-11-2022 09:57
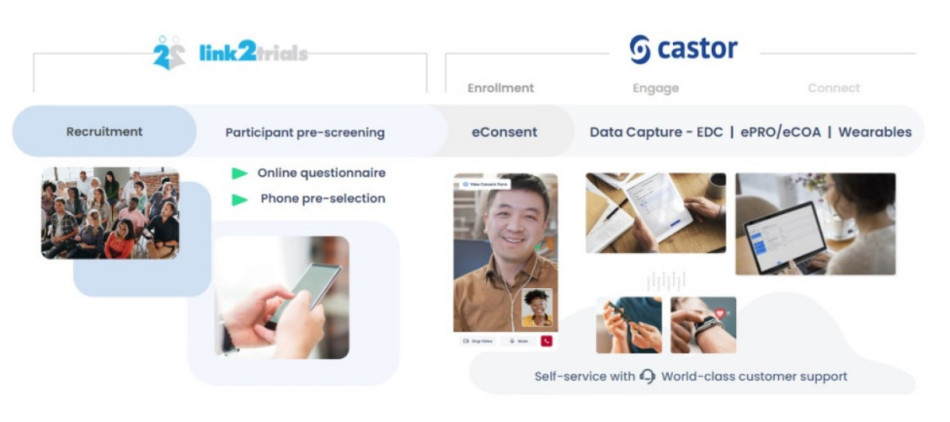
Partnerships are a key to success when it comes time for your company’s next clinical trial. In this blog post, we will illustrate how building an effective partnership ecosystem can speed up timelines while optimizing operational activities in a clinical trial while highlighting a recent success story between Essity, Link2Trials, and Castor.
How to integrate partnerships to...Read more
PRESS RELEASE: clinical study for our lead product, pHyph, a vaginal tablet for topical treatment of bacterial vaginosis (BV), completes recruitment!
Luis Eduardo Swart
2957

10-02-2022 11:46
"Really fantastic feeling to have finalized enrolment of the 150 patients in our placebo-controlled study in bacterial vaginosis #Nefertiti - What an achievement by the Gedea Biotech AB team, the sites, LINK Medical Research, Link2Trials - and what a milestone for us!"
Read the full press release on the Gedea Biotech AB website: Link
PRESS RELEASE: clinical study for our lead...Read more
Patient Site Liaison (PSL)
Tamara Schouten
2784

16-11-2021 11:46
Link2Trials uses its PSLs to ensure the potential trial participants we refer fit the study in and exclusion criteria.
My name is Tamara. I have been working as a Patient Site Liaison (PSL) at LinkTrials for several years now. As a PSL you are the link between a large number of applications and the actual (potential) participants for a clinical study. With a medically relevant background,...Read more
4 pointers for your post COVID-19 patient recruitment activities
Simon Klaasen
4495
20-04-2020 09:56
In most countries, regular care will be one of the first things to re-start post-Covid-19. With that, some of our clinical trials can re-start as well. We have discussed the re-start of trial recruitment with a lot of sites for a lot of studies in different countries and indications. This resulted in 4 pointers, for you to ensure the re-start of your trials and trial recruitment is as smooth as...Read more
PreCare Trial & Recruitment wins the European Site Patient Recruitment Innovation Award (SPRIA EU) with a Link2Trials patient informed consent video
Luis Eduardo Swart
6200
13-04-2018 10:30
PreCare Trial & Recruitment wins the European Site Patient Recruitment Innovation Award (SPRIA EU) with a Link2Trials patient informed consent video
Last week at the 2018 Site Solutions Summit, PreCare Trial & Recruitment was awarded the SPRIA EU prize for their Informed Consent Form (ICF) video developed by Link2Trials. The video informed potential participants thoroughly about...Read more
What is wrong with your online clinical trial information?
Simon Klaasen
6198

07-10-2016 13:19
You ran a very successful online advertisement campaign for your clinical trial and you can see that many people have visited your clinical trial page. Unfortunately, very few follow through on their initial interest. Does this sound familiar to you? Then there is definitely something wrong with your online clinical trial information.
At first glance, your advertisement campaign seems to be in...Read more
Patient Recruitment; Eight Seconds To Take Off!
Simon Klaasen
7751

12-10-2015 21:16
According to a recent Microsoft report the average human attention span has dwindled from 12 seconds in 2000 to only 8 seconds in 2013. That is even less than a goldfish! These 8 seconds are very important because the average visitor of your website or portal will leave if he doesn’t find the information he is looking for in this very short timeframe.
It has already been mentioned in an...Read more
Patient centric recruitment; will technology make it happen?
Simon Klaasen
7404

06-10-2015 22:26
When reading about patient centricity one might get the impressions that technology will make it all happen. A lot of money is spent on portal solutions to make trial information more accessible for patients, and on mobile apps that will make it easier for patients to share the information the industry needs from them. But looking at these solutions the question pops to mind how all of this will...Read more
Online media rapidly taking over traditional media as news source
Aad Liefveld
12062

03-09-2015 14:59
According to data published by Zenithoptimedia, between 2010 and 2014 the average time people spent online grew from 60 to 110 minutes per day.
At the same time newspaper and magazine consumption dropped by 26% and 19%, respectively.
You will find more statistics at Statista
Moreover, according to a study by Reuters Institute for the Study of Journalism very few people consider print...Read more
5 Easy steps to find the right patients for your clinical trial
Aad Liefveld
6654

25-08-2015 07:44
Recently the National Cancer Institute in the US has published a concise and easy to read step-by-step guide for cancer patients who are looking for clinical trials. Each of the ten steps in the guide explains in simple terms what to do and if applicable where to find more detailed information.
The guide not only provides links to multiple sources containing information about ongoing and...Read more
Learn about social media and patient recruitment from your peers
Aad Liefveld
6465

28-07-2015 16:22
The roundtable discussion we facilitated in London last month, made us aware that there still is a lot of misunderstanding and concerns in the pharmaceutical industry about using social media as an effective tool for patient recruitment and patient centricity.
We also learned that there is a lot of interest for informal and interactive discussions with peers about this particular subject,...Read more
Join us at the 2015 Proventa Clinical Data Strategy Meeting Europe in London
Aad Liefveld
6305

25-05-2015 15:07
On July 1, 2015 Link2Trials will attend the Clinical Data Strategy Meeting Europe 2015 in London.
Simon Klaasen will facilitate a roundtable discussion about patient centricity, social media and patient recruitment based on the following statements:
Patient centricity, engagement and empowerment will increase patient participation in clinical trials
Social media can be a very efficient tool...Read more
Patient Centric Recruitment; a CRO’s Perspective
Aad Liefveld
8199

21-05-2015 08:18
Reasons for Change
It has all been said before: Over 95% of the clinical studies fail to meet their original time lines and budgets[1] and that one day of delay could cost a sponsor $1.6 million on averaged lost revenue.[2]
Between 2006 and 2016 the global spending on drug development will grow from $108 billion to over $148 billion.[3] Clinical trials account for 45% to 75% of this cost[4] and...Read more
Choose category
Latest posts
- 04 November, 2025
- Link2Trials Q&A_30
- 15 August, 2025
- Link2Trials Q&A_29
- 18 July, 2025
- Link2Trials Q&A_28
- 04 July, 2025
- Link2Trials Q&A_27
- 03 June, 2025
- Link2Trials Q&A_26

 Argentina
Argentina Australia
Australia Balgarija
Balgarija België
België Canada
Canada Česko
Česko Chile
Chile China (中国)
China (中国) Colombia
Colombia Danmark
Danmark Deutschland
Deutschland England
England España
España France
France Ireland
Ireland Italiana
Italiana Lietuva
Lietuva Magyarország
Magyarország Nederland
Nederland New Zealand
New Zealand Österreich
Österreich Polska
Polska Schweiz
Schweiz Singapore
Singapore Slovenija
Slovenija Slovensko
Slovensko Suomi
Suomi Sverige
Sverige United States
United States Israel
Israel


