Blog
A must-read for anyone who wants to understand what impact tailored behavioral interventions can have on adherence!
Simon Klaasen
1480
17-08-2022 12:43
A must-read for anyone who wants to understand what impact tailored behavioral interventions can have on adherence!
This is precisely why we have developed our adherence support services for clinical studies. Thank you for sharing your insights, Dr Katerina Kassavou, Chimweta Chilala and Professor Stephen Sutton of the University of Cambridge.
Read more
Read more
A model to predict adherence behavior
LUISA CARMO
1458

27-05-2022 21:05
At Link2Trials we are excited to be part of this latest initiative to develop a model to predict adherence behavior.
This model will help healthcare providers to better support patients and enable the pharma industry to develop better products and services.
What a great BEAMER team we have! So many wonderful, interesting and foremost focused and motivated people!...Read more
Patient Site Liaison (PSL)
Claire Davis
1710

08-04-2022 20:17
Link2Trials uses its PSL to ensure the potential trial participants we refer match the study in- and exclusion criteria.
Claire speaking:I’ve recently started working in a Patient Site Liaison (PSL) research role with Link2Trials. The role requires contacting patients who have expressed an interest online for participating in research studies relevant to themselves. I was attracted to the...Read more
Partnerships can streamline your clinical trial
Aad Liefveld
1540

12-05-2022 20:08
Did you miss the webonar about how partnerschips can streamline your next clinical trial from Xtalks?
Or did you join the live webinar but want to review the presentation, click below to watch the recording.
In this webinar Simon Klaasen, Sandra Tobisch and Derk Arts, discussed:
The importance of interoperability to reduce site and patient burden
Considerations for...Read more
Beamer
Aad Liefveld
1452

05-11-2022 19:50
Did you miss last week's BEAMER webinar?
Kicking off our BEAMER webinar series this month, we explore the scope of non-adherence with expert panelists from across the EU.BEAMERBEhavioral and Adherence Model for improving quality, health outcomes and cost-Effectiveness of healthcaRe
Click below and watch...Read more
Decentralized Clinical Trials
Marjolein Nankman
1362

28-03-2022 19:40
Heads up!
Looking for a seamless experience for patients within your decentralized clinical trial?Because of their patient-centric approach DCTs will address important patient needs that often go unmet in a traditional, site-based set-up. By nature DCTs make clinical trial participation less burdensome, enable a more representative patient access, help develop a stronger evidence package and...Read more
Breederode University course for research nurses
Marjolein Nankman
1332

25-03-2022 19:23
Breederode University of Applied Sciences Rotterdam provides a 1-year specific training course for research nurses/clinical trial coordinators to become experts in the field of setting up and supporting clinical trials.
Great to see that this course has a specific focus on patient recruitment. This afternoon Simon Klaasen will give a guest lecture and share his expertise in the field of patient...Read more
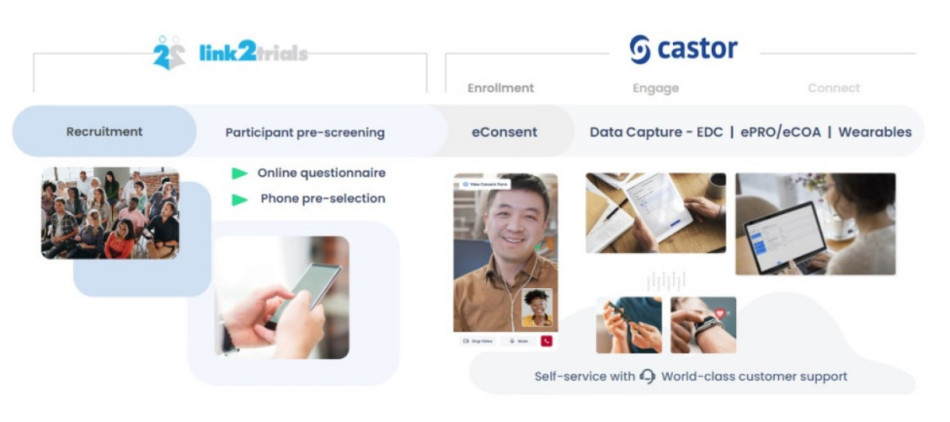
How to Improve Medical Device Clinical Trials With The Right Partner Ecosystem
Simon Klaasen
1705
01-11-2022 09:57
Partnerships are a key to success when it comes time for your company’s next clinical trial. In this blog post, we will illustrate how building an effective partnership ecosystem can speed up timelines while optimizing operational activities in a clinical trial while highlighting a recent success story between Essity, Link2Trials, and Castor.
How to integrate partnerships to...Read more
PRESS RELEASE: clinical study for our lead product, pHyph, a vaginal tablet for topical treatment of bacterial vaginosis (BV), completes recruitment!
Edwin Swart
1576

10-02-2022 11:46
"Really fantastic feeling to have finalized enrolment of the 150 patients in our placebo-controlled study in bacterial vaginosis #Nefertiti - What an achievement by the Gedea Biotech AB team, the sites, LINK Medical Research, Link2Trials - and what a milestone for us!"
Read the full press release on the Gedea Biotech AB website: Link
PRESS RELEASE: clinical study for our lead...Read more
Decentralized trials are here to stay.
Marjolein Nankman
1327

19-01-2022 11:46
Decentralized trials are here to stay!
Read more
Decentralized trials
Simon Klaasen
1538

13-01-2022 11:46
Not just for a pandemic: Decentralized trials "are here to stay, and they should be deployed in a fit-for-purpose manner"
Decentralized clinical trials are here to stay, and the long-term benefits outweigh the higher initial costs and technical challenges of remote...Read more
Patient Site Liaison (PSL)
Tamara Schouten
1432

16-11-2021 11:46
Link2Trials uses its PSLs to ensure the potential trial participants we refer fit the study in and exclusion criteria.
My name is Tamara. I have been working as a Patient Site Liaison (PSL) at LinkTrials for several years now. As a PSL you are the link between a large number of applications and the actual (potential) participants for a clinical study. With a medically relevant background,...Read more
BEAMER project; BEhavioral and Adherence Model for improving quality, health outcomes and cost-Effectiveness of healthcaRe
Aad Liefveld
1501

10-11-2021 11:46
It doesn't get more patient centric than this!BEAMER the latest EFPIA - European Federation of Pharmaceutical Industries and Associations Innovative Medicines Initiative (IMI) initiative is about finding a new and effective way to improve patient adherence to treatment.
BEAMER will focus on assessing and understanding patient needs. At Link2Trials we are honored to be part...Read more
Protocol deviations; tell-tale signs of patient non-adherence during clinical trials
Aad Liefveld
3926

25-05-2021 09:01
When discussing patient adherence during clinical trials, most people will say that on average it is high and that the issue of non-adherence really surfaces during clinical care. But is this true or are we missing something in clinical trials? I think we are missing something, and the issue is far greater than we think it is.
Early drop-out during clinical trials has always been and still is an...Read more
Losing touch with patients is not an option!
Aad Liefveld
4032

22-12-2020 17:02
In the past few months the healthcare industry has sped up the adoption and implementation of digital remote healthcare solutions to meet patients’ health-related preferences and at the same time overcome the challenges of social distancing and limited human interaction. The pharmaceutical industry is also moving forward on the subject of virtual and hybrid clinical trials.
The many...Read more
Join Link2Trials and EFPIA-IMI to improve patient adherence!
Aad Liefveld
3842

19-09-2020 11:05
Join Link2Trials and EFPIA-IMI to improve patient adherence!
It is official now! A few weeks ago we have received confirmation from the EFPIA Innovative Medicine Initiative (IMI) that Link2Trials has been accepted as Associated Partner for the patient adherence improvement project (Call 23, Topic 6).
Patient non-adherence to prescribed treatment is an issue that affects patient health outcomes...Read more
4 pointers for your post COVID-19 patient recruitment activities
Simon Klaasen
3463
20-04-2020 09:56
In most countries, regular care will be one of the first things to re-start post-Covid-19. With that, some of our clinical trials can re-start as well. We have discussed the re-start of trial recruitment with a lot of sites for a lot of studies in different countries and indications. This resulted in 4 pointers, for you to ensure the re-start of your trials and trial recruitment is as smooth as...Read more
How to communicate with patients more effective
Aad Liefveld
4307

12-12-2018 07:06
Today early drop-out still is a major challenge we must deal with when running clinical trials. The past few years patient centricity and patient engagement initiatives have generated major improvements. This is the second in a series of articles about a different perspective on the subject and a new and innovative approach to improve early drop-out rates.
Communicating information...Read more
It is all about expectations
Aad Liefveld
3657

05-12-2018 09:34
Today early drop-out still is a major challenge we must deal with when running clinical trials. The past few years patient centricity and patient engagement initiatives have generated major improvements. This is the first in a series of articles about a different perspective on the subject and a new and innovative approach to improve early drop-out rates.
Early Drop-out; still a major...Read more
Thank you; two words with tremendous impact but too little said during clinical trials
Aad Liefveld
3897

21-11-2018 19:26
A plea to express gratitude
Recently, I shared an editorial article from Nature on LinkedIn about the value and importance of true patient engagement in clinical trials. Involving patients from the earliest start of the clinical trial can and will lead to more effective and feasible clinical trial protocols. Patients are equivalent partners, and researchers need to incorporate patient feedback...Read more
PreCare Trial & Recruitment wins the European Site Patient Recruitment Innovation Award (SPRIA EU) with a Link2Trials patient informed consent video
Edwin Swart
4756
13-04-2018 10:30
PreCare Trial & Recruitment wins the European Site Patient Recruitment Innovation Award (SPRIA EU) with a Link2Trials patient informed consent video
Last week at the 2018 Site Solutions Summit, PreCare Trial & Recruitment was awarded the SPRIA EU prize for their Informed Consent Form (ICF) video developed by Link2Trials. The video informed potential participants thoroughly about...Read more
Why you should focus on retention
Aad Liefveld
5970

11-07-2017 19:39
Growing costs of drug development
Over 95% of the clinical studies fail to meet their original time lines and budgets.[1] Between 2006 and 2016 the global spending on drug development grew from $108 billion to over $148 billion.[2] Clinical trials account for 45% to 75% of this cost[3] and in 2012 the life sciences industry was already spending $1.2 billion[4] on patient recruitment alone!
It...Read more
What is wrong with your online clinical trial information?
Simon Klaasen
5132

07-10-2016 13:19
You ran a very successful online advertisement campaign for your clinical trial and you can see that many people have visited your clinical trial page. Unfortunately, very few follow through on their initial interest. Does this sound familiar to you? Then there is definitely something wrong with your online clinical trial information.
At first glance, your advertisement campaign seems to be in...Read more
Join our next webinar: Why social media has to be part of your patient recruitment strategy
Aad Liefveld
5243
20-09-2016 17:56
Online patient recruitment has proven to be a valuable strategy for finding the right subjects for the majority of the clinical trials. The very large online population accessible on the many social media platforms and the ability to specifically target the study population in the advertisement campaigns make online recruitment the tool of choice.
Join our next webinar and learn why social media...Read more
Patient Recruitment; Eight Seconds To Take Off!
Simon Klaasen
6573

12-10-2015 21:16
According to a recent Microsoft report the average human attention span has dwindled from 12 seconds in 2000 to only 8 seconds in 2013. That is even less than a goldfish! These 8 seconds are very important because the average visitor of your website or portal will leave if he doesn’t find the information he is looking for in this very short timeframe.
It has already been mentioned in an...Read more
Choose category
Latest posts
- 05 February, 2025
- How to Recruit Patients for Clinical Trials: A Comprehensive Guide
- 03 July, 2024
- Link2Trials Q&A_18
- 07 June, 2024
- Link2Trials Q&A_17
- 23 April, 2024
- Link2Trials Q&A_16
- 06 February, 2024
- Link2Trials Q&A_15

 Argentina
Argentina Australia
Australia Balgarija
Balgarija België
België Canada
Canada Česko
Česko Chile
Chile China (中国)
China (中国) Colombia
Colombia Danmark
Danmark Deutschland
Deutschland England
England España
España France
France Ireland
Ireland Italiana
Italiana Lietuva
Lietuva Magyarország
Magyarország Nederland
Nederland New Zealand
New Zealand Österreich
Österreich Polska
Polska Schweiz
Schweiz Singapore
Singapore Slovenija
Slovenija Slovensko
Slovensko Suomi
Suomi Sverige
Sverige United States
United States Israel
Israel


